

Diamagnetism is based on the interaction between electrons and the magnetic field. Since all materials contain electrons, all materials are diamagnetic. Paramagnetism is due to permanent magnetic moments of atoms. Not all types of atom have magnetic moments, so this mechanism is not universal. Ferromagnetism arises when these permanent magnetic moments are strong enough to interact and influence each other. This collective phenomenon is several orders of magnitude stronger than the other magnetic interaction mechanisms, which are based on the electronic charges or magnetic moments of individual atoms only.
According to Lenz's law, any current induced by a magnetic field gives rise to a magnetic field opposing the original inducing field. This follows from the application of the right-hand rule both on induction of a current by a field and vice versa. For this reason, diamagnetic susceptibility is always negative, i.e. the density of B-field lines is reduced by diamagnetism.
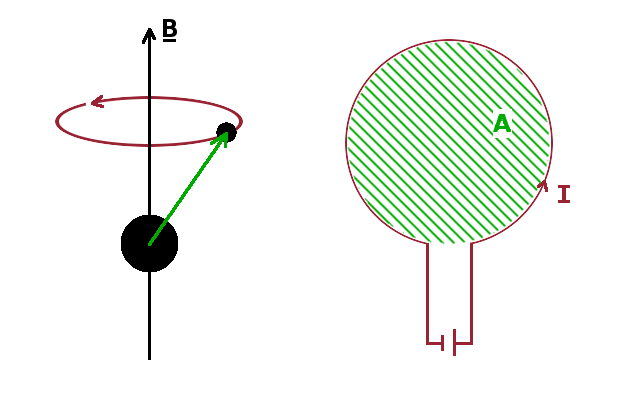
In a semi-classical view of an atom (left figure), an electron can be regarded as orbiting the nucleus at a fixed distance. If placed in a magnetic field, a precession of the vector linking the electron with the nucleus around the field axis is observed. The frequency $\omega$ of that precession is given by the Larmor theorem: $$\omega=\frac{eB}{2m_e}$$ where $e$ and $m_e$ are the electron's charge and mass, respectively. Given that a current is the number of charges flowing through a point per unit time, the current $I$ flowing in an atom due to the Larmor precession is $$I=-Ze\frac{\omega}{2\pi}=-\frac{Ze^2B}{4\pi m_e}$$ where the factor $-Z$ adds up all the electrons in the atom and acknowledges the fact that electrons have a negative charge.
Given a current, we we can determine the magnetic moment induced by it. For this purpose, it helps comparing the Larmor current in the atom with a current flowing around a single wire loop (right figure). The magnetic moment $p_m$ induced by a current $I$ is proportional to the current and the area enclosed by it, $p_m=AI$ (and pointing in the direction normal to that area). For a circular loop, $A=\pi r^2$, and $$p_m=\pi r^2I=-\frac{Ze^2B}{4m_e}\langle r^2\rangle$$ where $\langle r^2\rangle$ stands for the median distance of all the electrons from the field axis in the case of the atom.
This fairly crude classical interpretation gives us a good estimate of the induced magnetic moment in an atom due to the interaction of the material's electrons with the magnetic field. Given the chemical composition and density (and hence electron density) of a material, we can calculate its diamagnetic susceptibility. This simple model produces adequate results except in the case of conduction electrons in metals. Since they are delocalised over many atoms within the lattice, the idea of a Larmor current doesn't do them justice.
For
paramagnetism,
a quantum-mechanical analysis is needed. The
magnetic moment, $\vec{p}_m$,
of an atom depends on its
total angular momentum, $\vec{J}$.
The two are linked by the
gyromagnetic ratio, $\gamma$, (in Hz/T),
an element-specific material constant:
$$\vec{p}_m=\gamma\hbar\vec{J}$$
Alternatively, we can express the link between $\vec{p}_m$ and $\vec{J}$ in terms of multiples of the
Bohr magneton
$$\mu_B:=\frac{e\hbar}{2m_e}$$
which is essentially the "quantum of magnetic moment" (measured in J/T). Using this formalism, the magnetic moment is
$$\vec{p}_m=-g\mu_B\vec{J}$$
The
g-factor
(dimensionless) depends on how the various spins and orbital angular momenta of all the electrons in the
atom couple. Its value can be calculated using the
Landé equation:
$$g=1+\frac{J(J+1)+S(S+1)-L(L+1)}{2J(J+1)}$$
where $S$, $L$ and $J$ are the combined
spin, orbital and total angular momentum quantum numbers
of the electrons in the atom.
If the angular momenta combine in such a way that the g-factor is zero, the material cannot be paramagnetic.
(More about how these
angular momenta
combine to follow in the Atomic Physics lecture.)
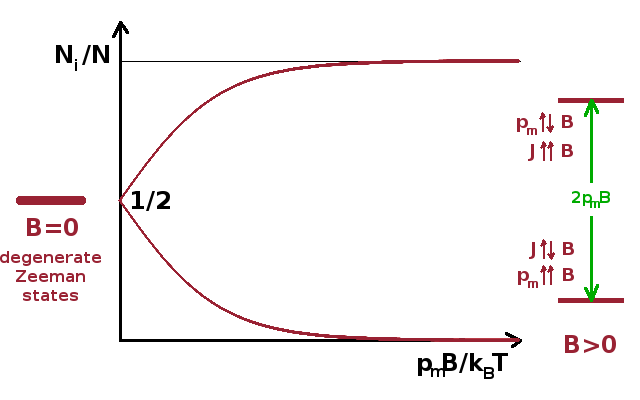
When an atom with a permanent magnetic moment is placed in a magnetic field, the previously degenerate magnetic states split into sub-states with distinct energy, separated by the energy of the magnetic interaction, $2p_mB$ (left and right margins of the figure). This is analogous to the Zeeman effect separating the energy levels of spin states of electrons, except that we consider the total angular momentum (including spin and orbital components) of the atom as a whole. The magnitude of the Zeeman splitting is: $$E_{mgn}=-\vec{p}_m\vec{B}=m_{J}g\mu_BB$$ where the magnetic quantum number, $m_{J}=-J,-J+1,\cdots,+J-1,+J$, relates to the total angular momentum.
The relative population of the individual levels is given by the Boltzmann distribution, which compares the magnetic interaction energy $p_mB$ with the thermal energy $k_BT$, i.e. for a two-level system ($J=\frac{1}{2}$)
$$\frac{N_i}{N}=\frac{\exp{\left(\frac{p_mB}{k_BT}\right)}}{\exp{\left(-\frac{p_mB}{k_BT}\right)}+\exp{\left(\frac{p_mB}{k_BT}\right)}}=\frac{{\rm e}^x}{{\rm e}^{-x}+{\rm e}^x}\qquad\qquad x=\frac{p_mB}{k_BT}\qquad.$$
In a two-level system, the magnetisation is proportional to the excess population in the lower level, since only those excess moments aren't balanced by moments in the upper level, which point in the opposite direction: $$M=(N_1-N_2)p_m=Np_m\left(\frac{{\rm e}^x-{\rm e}^{-x}}{{\rm e}^x+{\rm e}^{-x}}\right)$$ The bracket is equal to $\tanh(x)$, and as long as $x\ll 1$ (i.e. if the magnetic interaction is much smaller than the thermal energy, $p_mB\ll k_BT$) $\tanh(x)\approx x$. This leaves: $$M=Np_mx=\frac{Np_m^2B}{k_BT}$$
This shows that the paramagnetic susceptibility $\chi_{para}$ scales inversely with temperature. This observation is known as the Curie law and the constant of proportionality is the Curie constant.
If $J\gt\frac{1}{2}$, there will be more than two Zeeman levels. Since their energies are always distributed symmetrically about the degenerate energy level at $B=0$, the analysis remains valid and there are simply more Boltzmann terms to sum in the denominator when the relative populations are calculated.
There are different scenarios by which spins and orbital angular momenta can combine to a total angular momentum (the LS and JJ schemes), depending on the type of atom. Naturally, this will affect the number of Zeeman states for the magnetic moment of the atom and thus the Curie constant of a material containing this atom. To complicate matters, if some electrons are in excited electronic states, this will also change J and thus affect the material's Curie constant.
Following these general observations on paramagnetism, we need to look into the effect of conduction electrons on magnetic properties of materials, which distorts the picture to some extent.