

The terms structure and morphology are often used together, and their meanings overlap considerably. Strictly speaking, morphology is the study of the shape of things, but in materials science the word is often used to denote the arrangement of smaller units into larger ones. This reflects the observation that structure is hierarchical, with different patterns occuring on different length scales.
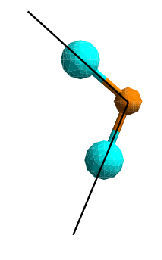
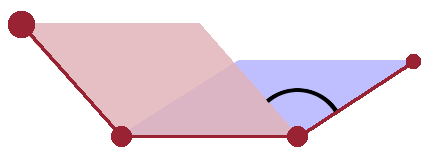
At the most fundamental level, structure arises from the way atoms of different elements bond together. Therefore, the smallest structural features are bond lengths and bond angles. These basic structural features result directly from electronic properties of the atoms involved and are therefore remarkably uniform, even in a disordered structure. Given that, in a condensed medium, atoms are generally densely packed, dihedral angles are an important structural feature that determines whether an ordered structure can be established or not. The dihedral angle is the angle between two planes spanned by the bond between two neighbouring atoms and their respective neighbours on opposite sides. In a crystal, dihedral angles involving the same species of atom are uniform, while an amorphous material or a liquid will have a wider distribution of dihedral angles. In the latter case these will also fluctuate on a short timescale, giving the liquid its flexibility.
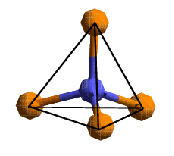
Each atom is typically surrounded by a fixed number of neighbours in a particular geometrical arrangement. This is described in terms of the co-ordination number, i.e. the number of nearest neighbours an atom has. The shape in which the neighbours are arranged is described in terms of co-ordination polyhedra. For many chemical elements, the co-ordination number and the type of co-ordination polyhedron found is the same whatever substance the atom is found in as it is determined by the electronic structure of the atom. For example, silicon atoms are virtually always surrounded by four nearest neighbours in tetrahedral co-ordination. On the other hand, some elements (in particular, transition metals) occur in different electronic configurations, leading to different co-ordination patterns.
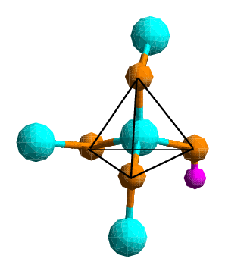
The co-ordination polyhedra are part of an extended structure, and each corner atom of one polyhedron usually belongs simultaneously to an adjacent polyhedron centred on another atom. This is described in terms of the connectivity of the structure.
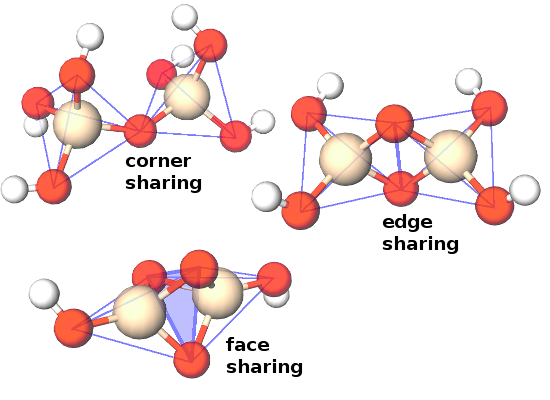
Co-ordination polyhedra are typically connected via shared corners, but shared edges and occasionally even shared faces occur, too.
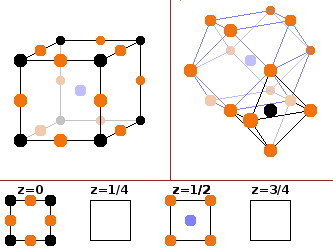
For periodic structures, an alternative description in terms of a crystal lattice is possible. In that case, a small repeat unit, the unit cell, is defined along with a description of the symmetry of the structure. As a result, the whole three-dimensional pattern can be generated by filling space with contiguous copies of identical unit cells. There is a finite number of periodic structures that can fill space without either gaps or overlaps. This allows a high level of abstraction from the chemical detail of the structure since different materials can have the same symmetry despite having very different bond lengths. In that case, they have the same crystal structure but with different lattice spacings. The figure on the right shows the same crystalline structure described in terms of its unit cell (left) and the co-ordination polyhedra surrounding its atoms (right).
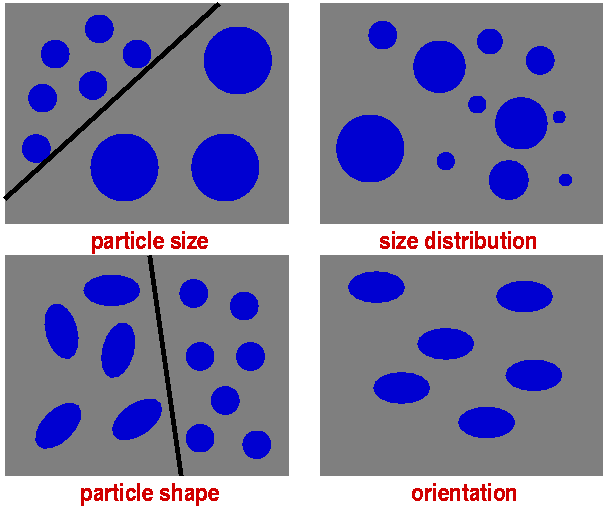
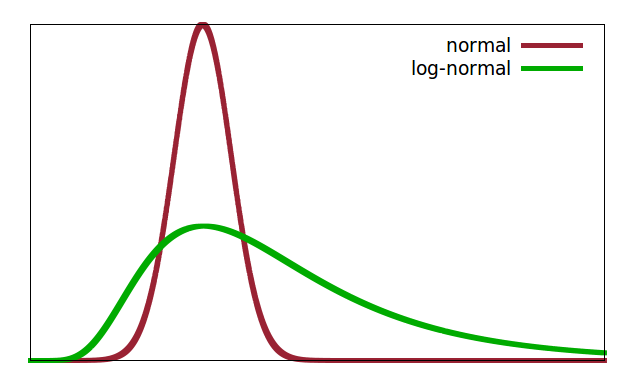
Leaving behind the structure immediately surrounding individual atoms leads to that aspect of the hierarchical structure often described as morphology. This includes, e.g., particles inside a material. This could be individual particles in a powder or conglomerate, a particulate phase embedded in a matrix of another composition (such as in a ceramic), or just the individual grains in a polycrystalline material, which are identical in composition and crystal structure but have different crystal orientation. Amongst the structural features of such a particulate system are the particle size and the particle shape. If all the particles in the sample have the same size, the sample is said to be monodisperse. More commonly, particle size is subject to a more or less wide distribution (polydispersity). The distribution could be Gaussian but is more usually found to have a long tail extending to much larger particles, often described in terms of a log-normal distribution.

If particles aren't spherical, they may be orientated other than randomly in a material. Orientation can have drastic effects on a material's properties (e.g. the cleavage of slate due to oriented platelets) and function (e.g. polarisation of light by aligned oblong particles).
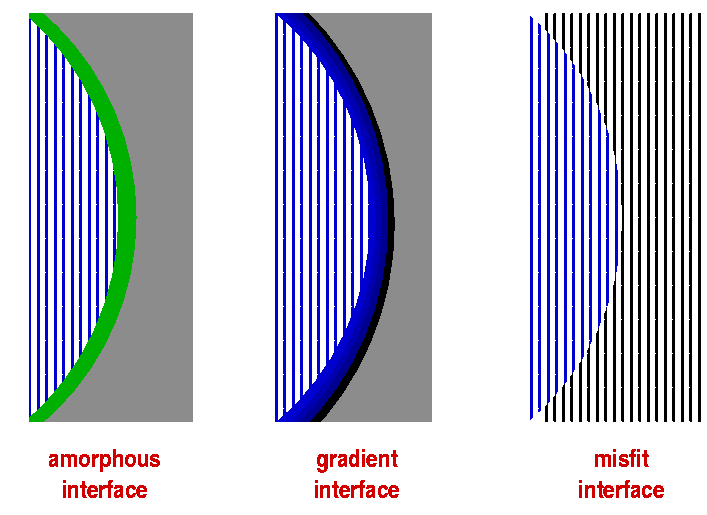
Where grains of different composition or crystal structure are in contact or particles are embedded in a matrix, interfaces become an important feature of the structure. For example, we have seen that grain boundaries are often disordered because of the orientational lattice mismatch of differently aligned grains. As a result of the disorder, it can be easier for ions to move along grain boundaries than to diffuse within grains. In nanocrystalline materials, where particles are only tens of atoms in diameter, the fraction of atoms located in distorted grain boundary environments can be substantial and lead to properties not seen in larger-grained versions of the same material. The interface part of the material effectively becomes a separate phase distinct from the bulk of the grains, sometimes termed the interphase.
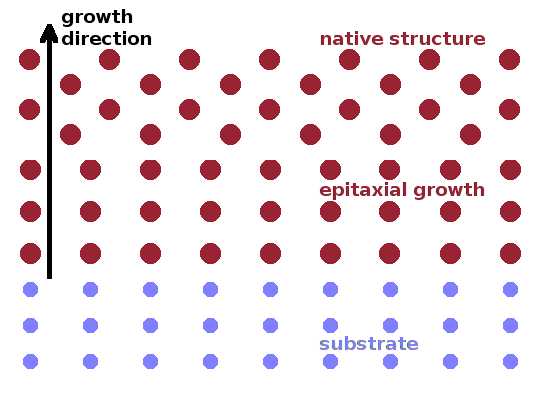
Where a material of one crystal structure is grown on a surface with a different structure, epitaxy is sometimes observed, i.e. the substrate structure is forced on the deposited material. This can persist for a few lattice planes before the bulk structure of the deposited material takes over. By doping with impurities it is possible to stabilise the epitaxial structure and essentially allow its infinite growth once it has been nucleated by the substrate. Where materials of different composition fuse together, it is possible to obtain a gradient structure where the chemical composition gradually morphs from one grain into the other without a sharp break. This gives the material added strength and is the mechanism by which sintered ceramics are produced. Sometimes the grain boundaries are so important for the performance of a material that their composition and structure is specifically targeted during manufacturing. Such grain boundary engineering techniques include e.g. pre-coating of particles before they are fused together, the use of electric or magnetic fields or extrusion techniques to induce particle orientation.
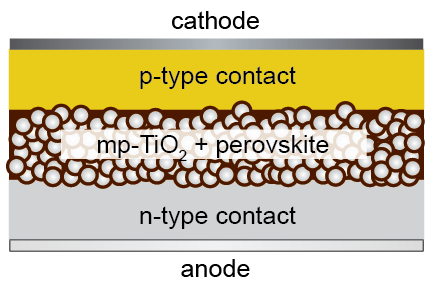
Devices such as photovoltaic cells are made up from a multitude of layers, each with their own internal structure. The performance of such multi-layer structures depends critically on the interfaces between the different layers. For example, in a thin-film solar cell, the internal surfaces must not refract light to such an extent that sunlight cannot reach into the active layer where charges are separated. If the charge separation layer contains a few very large grains, it is possible that the cell short-circuits. Therefore, each layer in a multi-layer structure needs to be designed and manufactured carefully and the interfaces between the layers must be controlled, too.
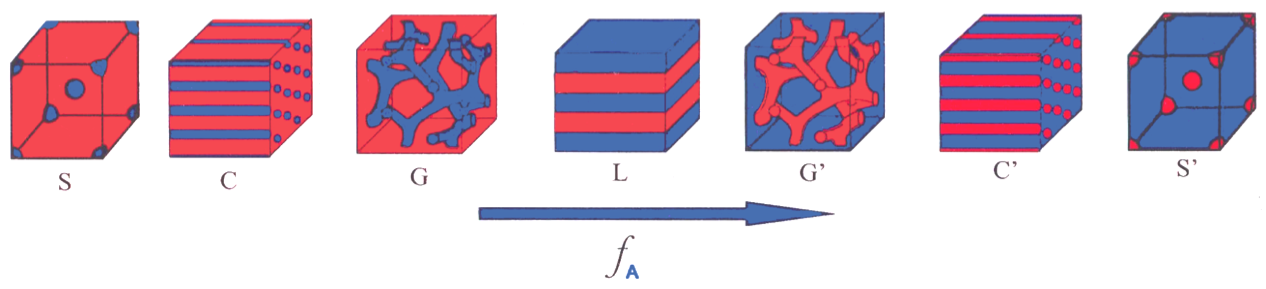
Besides such layer structures, morphologies with different dimensionality also exist. An example for this is the range of patterns observed in diblock co-polymers. A diblock molecule consists of two polymer strands with opposite chemical properties tied together. Since the two ends repel each other but cannot separate completely, patterns establish on a macroscopic scale depending on the relative size of the two blocks. This ranges from 0-dimensional micelles - isolated spheres of the minority component embedded in a matrix made from the majority component - via 1-dimensional cylinders, 2-dimensional lamellae - alternating layers of both components - to 3-dimensional bi-continuous phases where it is possible to move through the sample in all directions without ever having to cross a phase boundary, a feature known as percolation.
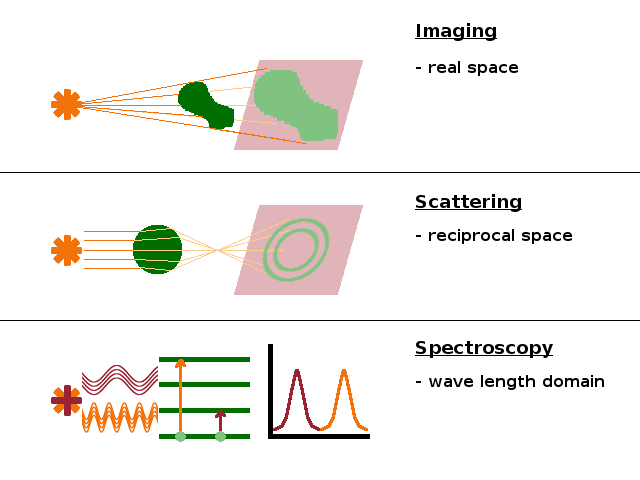
Given the broad range of structural features and length scales on which they occur, it is clear that there is no single experimental technique that can measure them all. Broadly speaking, there are three general classes of experimental techniques for structure determination: imaging, scattering and spectroscopy.
Most imaging techniques are based on the absorption of some of the probe medium (electromagnetic radiation or particle waves). The image is effectively the shadow cast by structures within the sample. In microscopy, optical elements such as lenses are used to magnify the image and bring out the detail. A special case is tomography, where a three-dimensional model is established from microscope images taken in different directions, effectively triangulating features inside the specimen.
Scattering techiques are based on interactions where a wave is refracted at internal surfaces (diffuse scattering) or diffracted by periodic structures in the sample (diffraction). These are indirect methods operating in reciprocal space and require Fourier transformation or modelling, but they provide much better statistics than what is achievable with direct microscopy techniques.
Finally, spectroscopy involves causing transitions between different states of atoms in the sample through interaction with electromagnetic radiation of different wavelength. In terms of structure determination, this is most useful in chemically heterogeneous samples.
All of these techniques can be used in transmission or reflection geometry. Alternatively, the probe or probing beam can be used in scanning mode to build up information about a heterogeneous sample from individual measurements of small volume elements.
While spectroscopy is covered in more detail in the Atomic Physics lecture, we'll begin this review of structural techniques by considering electron microscopy.