

Wave functions are standing waves of electron density: $\varrho_{el}=-e\psi(r)^{\ast}\psi(r)$. Since electrons are charged, this means a wave function represents an electric field. The electric component of the electromagnetic field from a dipole is a plane wave: $\vec{E}(t)=\left|E_0\right|{\rm e}^{-{\rm i}\omega t}\vec{u}_z$, where $\vec{u}_z$ is the unit vector in the direction of travel of the wave front. The two fields interfere when they intersect. The magnetic component of the dipole field also interacts with the electron wave function, but this interaction is much weaker.
The interaction is described in the form of a perturbation. The perturbing Hamiltonian consists of the dipole operator $-e\vec{r}$ and the oscillating electric field $E(t)$. The transition rate between the two states $\psi_1$ and $\psi_2$ involved in a transition triggered by this interaction is calculated according to Fermi's golden rule, i.e. the rate $U_{12}$ is proportional to the complex square of the integral over the interaction Hamiltonian sandwiched between the wave functions of the two states involved:
$$U_{12}\sim \left|\int\psi_2^{\ast}\;H_1\;\psi_1\;{\rm d}^3\vec{r}\right|^2 =e^2E_0^2\;\left|\int\psi_2^{\ast}\;\vec{r}\vec{u}_z\;\psi_1\;{\rm d}^3\vec{r}\right|^2$$
The ${\rm e}^{-{\rm i}wt}$ term from the electric field disappears because of the complex square. The scalar product $\vec{r}\vec{u}_z$ depends on the angle between the direction of travel of the electromagnetic wave (photon, ray...) and the principal orientation of the interacting state. For an s-state with spherical symmetry this makes no difference, but for a state with $l\gt 0$ (p,d,f,...) the average distance $|r|$ from the nucleus is different in different directions, so the transition rate becomes orientation dependent. Swapping the initial and final states makes no difference, i.e. $U_{12}=U_{21}$, which is what we'd expect based on the discussion of the Einstein coefficients.
The integral above has two contributing factors, a radial and an angular component, originating from splitting the wave function into its radial part $R_{n,l}(r)$ and spherical harmonics $Y_{l,m}(\varphi,\vartheta)$: $$\int\psi_2^{\ast}\;\vec{r}\vec{u}_z\;\psi_1\;{\rm d}\vec{r}=D_{12}I_{ang}\quad,\textrm{where}$$ $$D_{12}=\int_0^{\infty}R_{n_2,l_2}(r)\;r\;R_{n_1,l_1}(r)\;r^2\;{\rm d}r\quad\textrm{and}$$
$$I_{ang}=\int_0^{2\pi}\int_0^{\pi}Y_{l_2,m_2}^{\ast}(\vartheta,\varphi)\;\vec{r}\vec{u}_z\;Y_{l_1,m_1}(\vartheta,\varphi)\;\sin{\vartheta}\;{\rm d}\vartheta\;{\rm d}\varphi$$
Here, $D_{12}$ is the radial integral. It discriminates against transitions where $n_1$ and $n_2$ are very different, i.e. transitions between adjacent shells in the electronic structure are more likely than those where the electron's average distance from the nucleus changes a lot. Since radial wavefunctions are real, the need for a complex conjugate is removed; and the scalar product $\vec{r}\vec{u}_z$ collapses to the component aligned with the only variable, $r$.
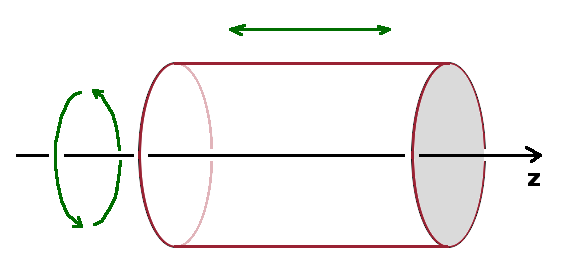
The angular integral, $I_{ang}$, is generally zero except if certain selection rules are met. These arise from the the orientation of the oscillating electric field with respect to the wave function. The electric field component of the interacting electromagnetic field can be split into a longitudinal oscillation along the forward-facing $z$-axis and two circularly polarised components with opposite polarisation in the $xy$ plane. For the oscillating component (called the $\pi$-component), the angular integral is $$I_{ang}^{\pi}=\iint Y_2^{\ast}\cos{\vartheta}\;Y_1\sin{\vartheta}\;{\rm d}\vartheta\;{\rm d}\varphi\quad.$$ Because of the cylindrical symmetry, $I_{ang}^{\pi}$ must remain unchanged after a full rotation about the $z$-axis, i.e. when changing $\varphi$ by $2\pi$. The part of $Y_1$ and $Y_2$ which affects $\varphi$ is the azimuth part, ${\rm e}^{-{\rm i}m\varphi}$. Therefore, if $m_1=m_2$, the $\varphi$-dependence of $Y_1^{\ast}$ and $Y_2$ just cancel out, and only if this condition is met can a $\pi$-transition occur. This is known as the selection rule for $\pi$-transitions: $\Delta m=0$.
For $\sigma$-transitions arising from the two circularly polarised components of the electric field, a slightly different selection rule applies. The angular integral here is $$I_{ang}^{\sigma}=\iint Y_2^{\ast}\sin{\vartheta}\;{\rm e}^{{\rm i}\varphi}\;Y_1\;\sin{\vartheta}\;{\rm d}\vartheta\;{\rm d}\varphi\quad.$$ The additional ${\rm e}^{{\rm i}\varphi}$ term means that the condition that the integral must be unaffected by a full turn of $\varphi$ is met when $\Delta m=\pm 1$, so this is the selection rule for $\sigma$-transitions.

The parity transform is a symmetry operation by which each point of an object is reflected by an inversion centre. That means its coordinates change from $\vec{r}$ to $-\vec{r}$. In practice that entails a reflection ($\vartheta\rightarrow\pi-\vartheta$) and a rotation ($\varphi\rightarrow\pi+\varphi$). Wave functions of s-type ($l=0$) have even parity (i.e. don't change sign under inversion). Each time $l$ is incremented by 1, the parity flicks to odd and back. As the series of figures shows, for $l=0$ and $l=2$, the sign of the wave function at the point indicated by the orange dot and its equivalent after inversion at the nucleus (purple dot) is the same, while for $l=1$ the sign is reversed. This means that the parity of a wavefunction goes with $(-1)^l$, and the vector $\vec{r}$ in the angular integral $I_{ang}$ transforms to $-\vec{r}$. Therefore, the overall parity of $I_{ang}$ is $(-1)^{l_2+1+l_1}$, i.e. the angular integral doesn't change if $l_2+1+l_1=0$ or $\Delta l=\pm 1$. This is the parity selection rule.
In addition to these selection rules, there are others for different types of interaction (e.g. magnetic) and system (e.g. rotational excitations in molecules).
Taken together, the selection rules for electronic transitions due to interaction with electromagnetic waves (radiation, photons...) are:
$$\Delta n=\textrm{any};\qquad\Delta l=\pm 1;\qquad\Delta m=0,\pm 1\quad.$$
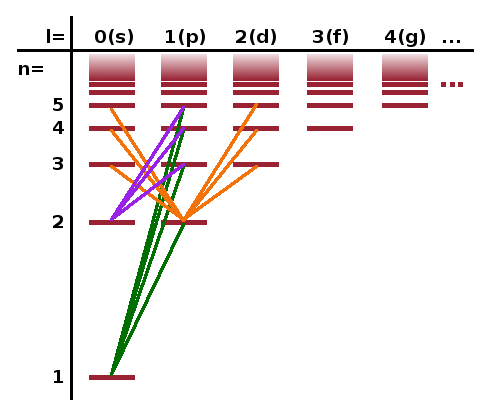
Transitions between states where the quantum numbers $l$ and $m$ change in this way are allowed transitions. A diagram showing the allowed transitions for a collection of states is called a Grotrian diagram. The Grotrian diagram here (not to scale) shows the allowed transitions from the states with $n=1$ and $n=2$ to states up to $n=5$ for hydrogen. The parity selection rule prohibits direct transitions to low-symmetry states with high $l$ values. Also, note that the 2s state has no downward transitions (to 1s) for the same reason. That makes the 2s state quite long-lived (metastable) even though it more energetic than the ground state.
Transitions that would not fulfil the selection rules are known as forbidden transitions. While these can occur, their transition rates are orders of magnitude lower. The reason they occur at all lies in the assumptions we've made. For example, we've only considered the electric part of the electromagnetic field of a photon; for the magnetic part the radial integral is much smaller and the selection rules are different. We've also used first-order perturbation theory. The higher orders are much weaker, but they can give rise to a small but finite transition rate for transitions that are forbidden to first order.
We've now established how electronic transitions between states come about through interaction with light (electromagnetic radiation, photons...). The electronic structure is a little more complex than what we expect from the hydrogen Schrödinger equation alone. Next we'll look at the fine structure of hydrogen.