
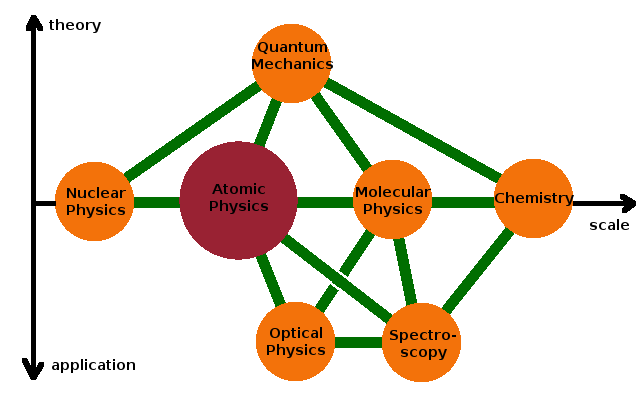
Atomic Physics is the branch of science that deals with the structure of the electron cloud within atoms. It regards the nucleus of the atom as a point charge of certain mass, without making any assumptions about its structure, which is the subject of Nuclear Physics. At the other limit of its characteristic length scale, atomic physics is neighbour to Molecular Physics and, in a wider sense, Chemistry, i.e. the interaction of atoms with each other and the structure of the resulting molecular electron systems.
All of these subjects draw heavily on Quantum Mechanics for their theoretical foundation because the states electrons can occupy in an atom or molecule are different solutions of the Schrödinger equation. The transitions between these states can be exploited to measure the electronic structure (and therefore for chemical analysis) using a variety of experimental techniques belonging to the field of Spectroscopy. The related field of Optical Physics deals with techniques to modify the populations of various electronic states, resulting in applications such as lasers. Both are based on the interaction between electromagnetic radiation and electron systems.
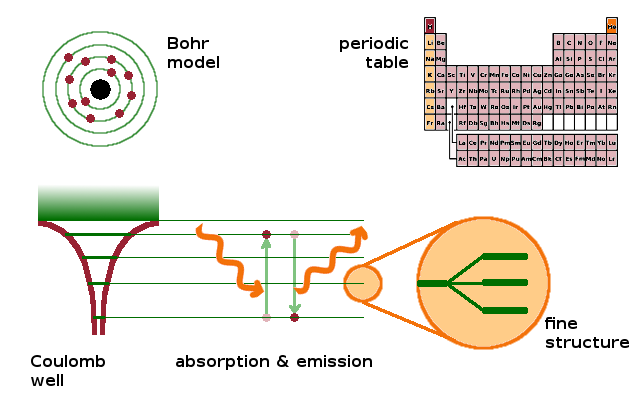
There are three different aspects to atomic physics: the electronic structure of atoms, spectroscopic techniques to measure electronic transitions between different states, and the variety of chemical elements represented by the periodic table and their properties, which are governed by their electronic structure.
By solving the Schrödinger equation for a quantum mechanical system such as an atom, we can determine which states an electron can occupy, and what the total energy of each state is. This gives rise to the shell structure of the atom, which had already been anticipated in the earlier Bohr model of the atom. Given distinct states with different energies, it follows that the excitation of an electron from one state to another requires a certain amount of energy, which can be supplied by a photon of the appropriate energy, $E=h\nu$. By sweeping through the electromagnetic spectrum, all possible transitions between energy levels can be mapped, including small splittings between levels of almost identical energy, i.e. the fine structure of the spectrum.
While the states of the hydrogen atom can be calculated analytically, the complexities of other atoms containing more than one electron mean that approximations are required. In practice, layers of complexity are built up gradually, from hydrogen via helium and the alkali metals to the rest of the periodic table, using perturbation theory. This approach uses the known states from the simpler system and adds a perturbation to adapt them (and their energies) to the more complex situation.
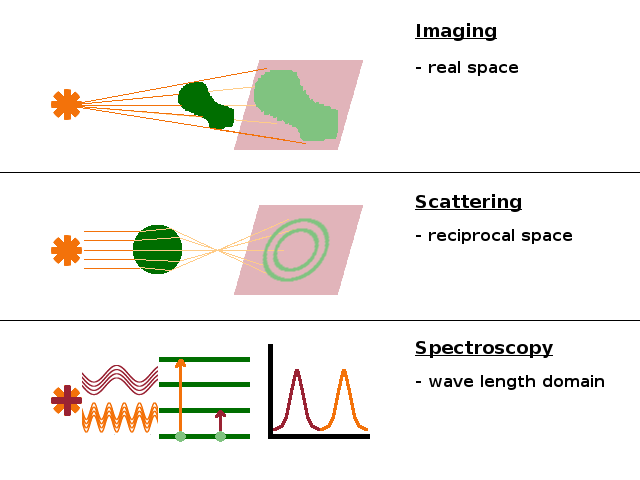
Much of experimental physics consists of various interactions between light and matter. Broadly, three different types of interaction can be distinguished, with a host of experimental techniques within each of these groups:
Imaging is a measurement in real space (units: metres), where the capacity of a material to absorb light is utilised. The material absorbs some of the incident photons, resulting in a shadow representing the shape and internal structure of the object. Microscopy is one flavour of this technique, and so is tomography, where images are taken from different angles to reconstruct a 3D model of the structure.
Scattering is a measurement in reciprocal space (units: $\mathrm{m^{-1};}$ in practice usually $\mathrm{nm^{-1}}$ or $\mathrm{{\AA}^{-1}}$), where the deflection of rays due to refraction at internal surfaces is probed. This changes the direction of propagation of the wave front, i.e. the wave vector, $\vec{k}$, changes direction. Scattering data are usually plotted in terms of either scattering angle or momentum transfer from the incoming wave to the scattering object. Both are in the reciprocal space domain, but sometimes scattering data are analysed by Fourier transformation from reciprocal into direct (i.e. real) space.
Spectroscopy is a measurement in the energy domain (units: J; in practice often eV) or, equivalently, the wavelength or frequency domain, where the absorption or emission of a sample is measured while either sweeping the wavelength of the source through an extended range or by using a white (polychromatic) source and analysing the response from the sample after the interaction has taken place in terms of the energy of the radiation leaving the sample. Absorption occurs when some of the incident photons are used to supply the energy for transitions between levels in the electronic structure of the material.
Of course the underlying physical processes exploited by these techniques all occur simultaneously whenever light and matter interact; it is merely a matter of choice of apparatus which type of interaction we choose to monitor and analyse.
The experimental side of atomic physics - atomic spectroscopy.