

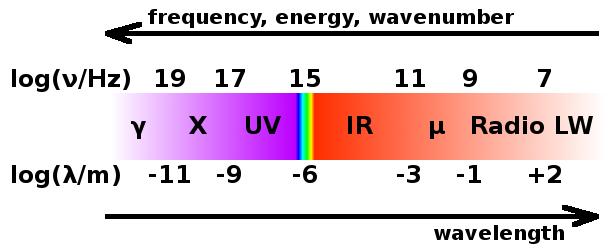
The electromagnetic spectrum spans over 15 orders of magnitude of photon energy. The visible part of that range comprises much less than one order of magnitude. For reasons of convenience within each field, different units are used for techniques using different parts of the spectrum. Since the whole spectrum consists of photons, i.e. particles without a rest mass, frequency and wavelength are linked by the speed of light, and the total energy is linked to both via Planck's constant:
$$\nu\lambda=c\qquad\qquad E=h\nu=\frac{hc}{\lambda}$$
Sometimes the spectroscopic wavenumber, $\tilde{\nu}=\frac{1}{\lambda}$, is used instead.
In order of increasing wavelength (i.e. decreasing frequency and energy), the spectrum consists of the following ranges (each shown with units used by convention - note these may be energies, wavelengths, frequencies or wavenumbers!)

A useful comparison is the frequency of radiation corresponding to the thermal energy of an object, i.e. the peak of the black body radiation curve for a given temperature. Thermal equilibrium demands that $$h\nu=k_BT\qquad,$$ so dimensional analysis produces, for ambient temperatures, a frequency of $$\nu=\frac{k_BT}{h}\sim\frac{10^{-23}10^{2}}{10^{-33}}{\textrm Hz}=10^{12}{\textrm Hz}$$ or $$\lambda=\frac{c}{\nu}\sim\frac{10^8}{10^{12}}{\textrm m}=10^{-4}{\textrm m}\quad,$$ which is in the infrared as one might expect for thermal radiation.
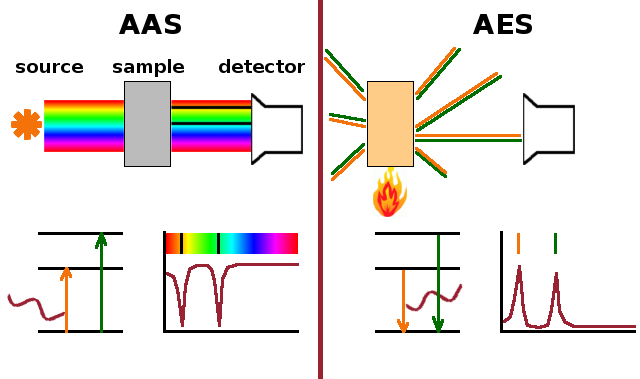
The two main techniques of atomic spectroscopy are Atomic Absorption Spectroscopy, AAS, and Atomic Emission Spectroscopy, AES. Both produce spectra whose lines reveal the spacing between energy levels in the electronic structure of the atoms in the sample. In AAS, the spectrum of light transmitted through the sample is analysed. Photons which match an allowed transition in the atom will be absorbed, giving rise to characteristic absorption lines in the spectrum. In AES, on the other hand, some electrons in the sample are excited into higher levels by an external source of energy such as a flame or, more commonly in modern instruments, an inductively coupled plasma (ICP) discharge. When the excited electrons return to their original state, they will emit a photon which is detected, producing emission lines at the same frequencies where the absorption lines occur in AAS. As a result, AAS and AES spectra are basically mirror images. AAS maps the ground state, so there will always be plenty of electrons available to absorb photons of the right frequency. This can produce high levels of noise, which AES doesn't suffer from. However, as AES requires electrons to be excited before they can emit, selective excitation can be a problem, potentially resulting in distorted spectra.
AAS and AES are terms usually used for spectroscopy using visible light. For most elements, the transitions in this spectral range are between electronic states in the outer shells of the electron system. In order to probe core levels of the atomic structure, higher energies are needed, and analogous x-ray spectroscopic techniques are used for this purpose.
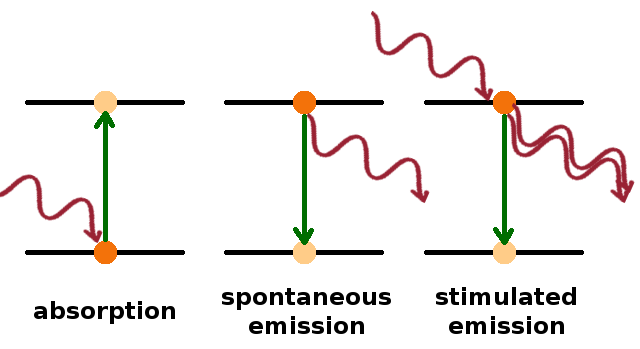
Transitions between any two energy levels involve photons whose energy is the difference between the energies of the two levels, i.e. $h\nu=E_2-E_1$. Besides the absorption process, there are two different emission processes: Spontaneous emission is the reverse of absorption, i.e. an electron drops from an excited state to one with lower energy and a photon is emitted, while stimulated emission is an emission event triggered by an incoming photon of the same frequency (this is the process by which a laser produces coherent light, as the emitted photon is in phase with the stimulating one).
Whether a particular transition occurs or not depends on the availability of an electron capable of making the transition, in the case of absorption and stimulated emission on the availability of a photon of a suitable frequency, and on a conditional probability that the transition will occur given these 'ingredients' are available. The conditional probabilities are called Einstein coefficients, $A_{21},B_{12},B_{21}$, and there is one for each of the three processes. The actual transition rates are therefore:
where $N_{1,2}$ is the population of the initial state and $\rho_{\nu}$ is the spectral density, i.e. the energy contained in all the photons of a particular frequency in the incident light: $\quad\rho_{\nu}=N_{\nu}h\nu$, where $N_{\nu}$ is the number of photons with frequency $\nu$.
There are alternative definitions of the spectral density. The only way to be certain that formulae including the spectral density are correct is to check the units.
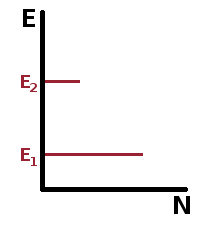
In thermal equilibrium, the populations do not change overall, so the number of absorption processes must equal the number of emission processes: $$N_1\rho_{\nu}B_{12}=N_2A_{21}+N_2\rho_{\nu}B_{21}\quad.$$ By solving this for the spectral density, we get $$\rho_{\nu}=\frac{N_2A_{21}}{N_1B_{12}-N_2B_{21}}=\frac{\frac{A_{21}}{B_{21}}}{\frac{N_1}{N_2}\cdot\frac{B_{12}}{B_{21}}-1}\quad.$$ Since we started with thermal equilibrium, the population ratio is given by the Boltzmann distribution, $\frac{N_1}{N_2}={\rm e}^{\frac{E_2-E_1}{k_BT}}$. Inserting that into the spectral density formula above leaves $$\rho_{\nu}=\bbox[lightgreen]{\frac{A_{21}}{B_{21}}}\left(\frac{1}{\bbox[yellow]{\exp\left(\frac{E_2-E_1}{k_BT}\right)}\frac{B_{12}}{B_{21}}\bbox[yellow]{-1}}\right)\quad.$$ Compare this with the spectral density of a black body: $$\rho_{\nu}=\bbox[lightgreen]{\frac{8\pi h\nu^3}{c^3}}\left(\frac{1}{\bbox[yellow]{\exp\left(\frac{h\nu}{k_BT}\right)}\bbox[yellow]{-1}}\right)\quad,$$ and it becomes clear that the two $B$ coefficients are always identical since $\bbox[yellow]{\frac{B_{12}}{B_{21}}=1}$, so absorption and stimulated emission always have the same conditional probability, and the population ratio between the two levels solely determines to which extent each happens. The ratio of the Einstein coefficients for spontaneous and stimulated emission on the other hand is the pre-factor from the black body equation: $\bbox[lightgreen]{\frac{A_{21}}{B_{21}}=\frac{8\pi h\nu^3}{c^3}}$. Note, however, that stimulated emission requires a photon while spontaneous emission doesn't, so the ratio of the actual rates of both emission processes is $\frac{A_{21}}{\rho_{\nu}B_{21}}$.
Spectroscopy measures the difference in energy between different electronic states. But why are there different well-defined states, and why is their energy different? We can find out by solving the Schrödinger equation of the simplest possible atom, the hydrogen atom.