If y1(x) and y2(x) are solutions of a differential equation, then any linear combination, i.e. any sum of the two solutions with linear prefactors, will also be a solution: y3=c1y1+c2y2. The eigenstates of the hydrogen atom are solutions of a differential equation, so any linear combination of these eigenstates will solve the Schrödinger equation just as well. Since we want to interpret the square of the new solution as a probability density, we have to choose the coefficients, c1 and c2, such that the wave function is normalised.
We will use the linear combination approach both to reorientate the atom's states to produce good overlap between the wave functions of neighbouring atoms (hybridisation) and then to produce the molecular states available to electrons once the molecule is formed (linear combination of atomic orbitals, LCAO).
As long as we are dealing with a single atom, the orientation of the reference frame in which the atom is described is irrelevant. All coordinates are relative to the centre of gravity of the atom. If the atom as a whole rotates, the reference frame rotates with it, so we won't notice. This is equivalent to our unawareness of the rotation of the Earth - we are tied to a reference frame that rotates, and all other objects around us are in the same reference frame.
When forming a chemical bond, the two atoms involved each come with their own reference frame attached. If atom 2 rotates, together with its frame, then this will be noticeable from atom 1, much as the rotation of the Earth is obvious to an observer on the moon, who isn't locked to the Earth's reference frame.
A chemical bond is an increase in electron density along or to the sides of the connecting line between the nuclei. The atomic states from the solution of the hydrogen atom have no preferential orientation. It is necessary to "repackage" the solutions and produce states which have a large absolute value of the wave function in the required direction, i.e. facing the neighbouring atoms with which chemical bonds are to be formed.
This is achieved by hybridisation, i.e. by linear combination of the states of the atom in such a way that the hybrid states have the electron density in the right place.
The example on the right shows the hybrid state 2sp, which is formed by linear combination of a 2s and a 2p state. The result is a wave function with a spread-out negative side on the left and a focussed positive lobe stretching out to the right. This would maximise the electron density in a chemical bond with an atom on the right. At the same time, this hybridisation process also produces an equivalent hybrid state where the two sides behave exactly opposite (i.e. where the linear coefficients have opposite sign). The number of hybrid states generated is always the same as the number of states making up the hybrid. Only states with (nearly) the same energy can be hybridised. This becomes clear when looking at the wave functions of states with different n: Their wave functions have maxima at very different distances from the nucleus, so they don't match very well.
Note that hybridisation is not a physical process but a mathematical technique, i.e. there is no physical change to the electron density within the atom occurring to prepare it to form a chemical bond. Hybridisation is merely repackaging the known states in a way that takes account of the orientation of the atoms with respect to each other by linear combination.
The same approach can be taken to generate the molecular states (or orbitals). Take two atomic orbitals (hybrids if appropriate) and get them to overlap. If the result is an increased probability density along (or near to) the connecting line between the nuclei of the participating atoms, we have a chemical bond.
The example on the right shows two atoms approaching along the axis of one of their 2p states. In the top row, the two lobes facing one another have the same sign; in the bottom row they have opposite sign. These are two different linear combinations of the same two atomic states. In the first example, the electron density increases between the nuclei, resulting in a bonding molecular state. In the second example, a very steep-sided node between the two nuclei causes all the probability density to face away from the atom opposite. This is an anti-bonding molecular orbital.
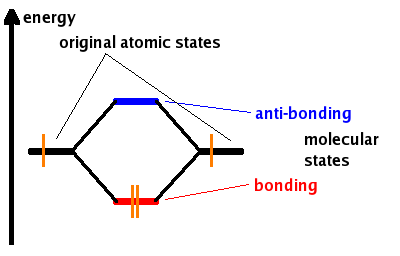
Both the bonding and anti-bonding states are generated at the same time - as always, the number of states obtained by linear combination is the same as the number of states put in. The energy advantage of the bonding state matches exactly the disadvantage of the anti-bonding state. Each state can be occupied by two electrons (of opposite spin). If both atomic orbitals are fully occupied, then both molecular states will be occupied and there is no net energy gain. If on the other hand (as in the Fig.) each atomic state contributes only one electron, then the electrons from both atoms can share the bonding molecular state. The result is a stable, i.e. energetically favourable, chemical bond.
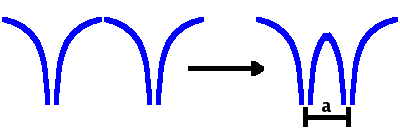
When the two atoms, represented as Coulomb wells, approach each other, the potential barrier between the two wells is gradually reduced. This allows electrons to occupy shared (delocalised) states with probability density at both atoms rather than only at the originating atom. For these electrons, the well size increases. The energy eigenvalues decrease with increased well size (cf. inverse-square relationship for the particle in a square well). This energetic advantage is what stabilises the chemical bond.
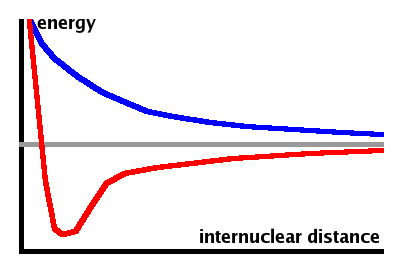
While the bonding state has an energy minimum at the internuclear distance where the reduction in energy due to the delocalisation of the electrons is balanced by the repulsive Coulomb interaction between the nuclei. The anti-bonding state has no such minimum; its energy is always higher than that of two separate atoms at infinite distance.
Both bonding and anti-bonding states always exist as both are linear combinations of the constituent atomic states. The bond is only stable if the bonding state is occupied by electrons while the anti-bonding state isn't. This is the case if the constituent atomic states contribute one atom each, i.e. are partially occupied themselves.